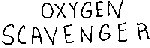Oxygen measurement techniques
DO Probe
Electrochemical method: Composed of two metal electrodes in contact with supporting electrolyte and separated from the test solution by a gas permeable membrane. A constant voltage is placed across the cathode and anode. Oxygen diffuses through the membrane and is reduced at the cathode by the voltage. This process produces a current flow, which is detected by the meter and is proportional to the partial pressure of oxygen. Since oxygen is "consumed" the sample needs to be stirred to ensure accurate readings.
Luminescence method: A fluorescent film is applied to the tip of a probe. The fluorescence is sensitive to the presence of oxygen. Changes in fluorescence activity are proportional to the concentration of oxygen in the sample. This technology has been used for years in the medical field. Those little fingertip oxygen sensors use the same technology. Other than greater stability and longer probe membrane life, one key advantage with "electro-optical" probes is that oxygen is not consumed; therefore stirring the sample is not required.
Advances in probe technology
Polarographic probes: single vs. dual thermistor
- Added thermistor to electrolyte (in addition to sample sensor). This allows temperature measurement in sample (water or air) AND in electrolyte. Air is poor heat sink (think of cooking in a copper vs. aluminum pot).
- People have tendency to calibrate too quickly when doing AIR calibrations.
- Double thermistor monitors differential between air and electrolyte and does not lock in calibration until the two are equal in temperature.
- Bottom Line: provides for more accurate & consistent calibrations.
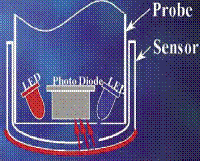
Luminescence DO technology
Initially Blue light from an LED
inside the probe excites luminescent
material on the sensor surface.
Image courtesy of HACH, Inc
Coming into vogue around 2005, luminescence represented a whole new technology for environmental chemistry, but which had been in use in medical field for many years. Blue light is transmitted to the probe sensor from an LED on the surface. This blue light excites the luminescent material which in turn emits red light as it encounters oxygen and is returned to its unexcited state. As oxygen molecules encounter the sensor surface, they react with the luminescent material, causing it to return to its un-excited state. This results in emission of red light which is correlated to oxygen content.
- Probes utilize a sensor coated with luminescent material.
- Blue light is transmitted to the sensor from an LED on the surface.
- This blue light excites the luminescent material which in turn emits red light as it returns to its unexcited state.
- The elapsed time from excitation till return to steady state is measured.
- The more oxygen present, the shorter the time it takes for red light emission.
- Time is measured and correlated to oxygen concentration.
Features of luminescence DO probe
molecules with the luminescent
material produces a red light proportional
to the oxygen concentration.
Image courtesy of HACH, Inc.
- Developed by the HACH company.
- Probe uses NO:
- anode (nothing to polish)
- cathode
- electrolyte
- membrane
- All of the items listed above are potential sources of error in conventional DO technology.
- Research shows luminescence requires:
- Less frequent adjustments during calibration
- Less maintenance
- Disadvantages of luminescence probe:
- Early model required an awkward funnel and magnetic stirrer.
- The funnel was difficult to use and posed a cross-contamination risk.
Luminescence technology is approved for wastewater compliance testing
- YES!
- Changes to ch. NR 219, Wis,. Admin. Code, which took effect June 1, 2009, included approved methodologies for Luminescence technology.
- Please note that other manufacturers offer this same technology under a different name (e.g., RDO or Rugged Dissolved Oxygen). The name may be different, but the technology itself, "electro-optical" or luminescence, is still approved.
DO Probe Maintenance
- Electrolyte replenishment
- Membrane failure
- Membrane rupture
- Membrane fouling
- Cathode and anode cleaning
- Follow manufacturer recommendations for interval and procedure
Troubleshooting DO probe problems
See the North Central Labs (NCL) website for solid information regarding diagnosing DO probe problems.
YSI 5100 on-board tools for monitoring membrane problems
- DO µA (micro-amps) should be from 8.0 to 17.0
- %/ µA (micro-amps) should be from 5.9 to 12.6 percent
- Replace membrane if outside this range
- Good tool for preventative maintenance
probe values for microamps(µA) and microAmp percentage (µA%).
General probe (non-LDO) maintenance
in& good condition (right). Note the light silver
appearance of the anode and the gold matte
finish of the cathode& on the right.
- Examine membrane daily.
- Replace membrane if air bubbles, wrinkles, or if there is buildup on or under the membrane.
- If gold tip (cathode) is tarnished, clean it.
- The gold should have a bright "matte" finish with fine scratches-DO NOT polish too much!
- Clean with sanding disk provided in the YSI cap.
- The silver anode should have a light silver color.
- Clean if anode is dark.
- Clean by soaking overnight in household ammonia solution.
- Rinse thoroughly with tap water, DI water and electrolyte.
Troubleshooting DO probe calibration problems
- Poor calibration may give the appearance of a dilution water problem when the water may be fine.
- Recommend calibrating using the air-saturated WATER calibration or Winkler titration.
- If air-saturated water calibration is used, use a good quality barometer in the laboratory.
- Check the barometer calibration against a reliable source at least quarterly (internet, airport, local station). Remember you must re-correct for actual altitude.
DO Probe malfunctions
Information obtained from North Central Laboratories
Troubleshooting probe membranes
- Allow ≥ 2 hr after membrane change for the probe to stabilize. Overnight is better.
- Warm-up instrument. Calibrate.
- Observe readings continuously for 2 mins. w/probe in bottle.
- Be sure the temperature is constant.
- Watch the readings carefully. Do NOT just record the initial reading and come back 2 minutes later. You need to actually see what happens over the time period.
- If readings drift slowly DOWN, a longer warm up time is required.
- If readings JUMP AROUND, the probe is not functioning properly.
- If readings STABLE in the air calibration bottle, sensor is probably OK.
- If readings stable in the air calibration bottle but not in solution, the membrane is probably defective.
Zero oxygen check (response check)
thoroughly. Sodium sulfite is
a significant oxygen scavenger,
so residue left behind will result
in oxygen depletion from samples.
- Dissolve 0.5-1 grams of sodium sulfite (Na2SO3) in 300 mL of lab reagent water.
- Stir slowly--avoid "tornadoes"; slowly pour into a BOD bottle.
- Calibrate your DO probe as you normally would.
- Place the probe into the "Zero Oxygen" solution.
- Observe!
- Meter should read "0" within two minutes.
- (With some older YSI systems, readings below 1.0 mg/l are considered zero.)
Copyright 2006. University of Wisconsin Board of Regents.
Unauthorized use prohibited without the expressed written consent of the UW, State Laboratory of Hygiene and the DNR Laboratory Certification & Registration Program.
- Contact information
- For information on the Lab Certification program, contact:
- Tom Trainor
Program chemist
Certification Services
DNR LabCert mailbox