BOD Basics
Five-day test
The BOD test originated in the United Kingdom due to pollution in the London area along the Thames River. They found that sewage dumped in the Thames River took five days to reach the ocean, hence the five-day incubation period. The test was officially adopted in 1908. The Royal Commission on Sewage Disposal, after adopting the BOD test also established the 30 mg/L standard which is used yet today in some permits.
The following are the critical steps in preparing and analyzing samples for BOD testing. The BOD test is a method-defined (empirical) technique. Consequently, the method must be followed carefully, and must be performed consistently from day to day and analyst to analyst. Potential errors can be encountered in each of the following steps in the BOD test.
- Prepare dilution water
- Prepare seed
- Perform the sample pre-treatment
- Determine dilutions required
- Measure out samples
- Fill bottles with dilution water
- Add seed to those that need it
- Top-off samples with dilution water
- Measure initial DO (DOi)
- Incubate 5 days (± 6 hrs)
- Measure Final DO (DOF)
- Determine BOD
- Evaluate the results of samples, GGA, blanks and duplicates and report.
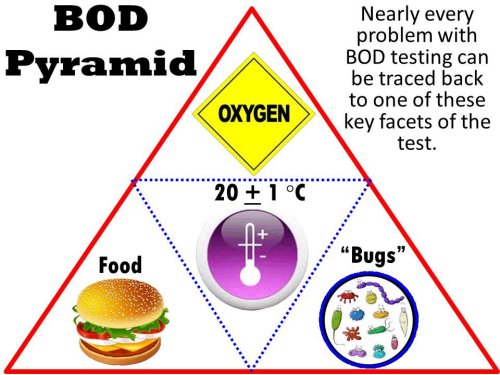
The BOD pyramid
At the heart of BOD testing is a concept that we call the "BOD Pyramid". Just as the food pyramid is the key to maintaining a healthy diet, the BOD Pyramid is the key to producing quality BOD results. Representing the three points of the triangle are the three critical aspects of BOD testing:
- a food source,
- a population of bugs, and
- available oxygen to be utilized by the bugs.
a food source that requires oxygen for biological degradation. A warm, constant temperature
environment in the dark is central of the pyramid and is the requirement for a hospitable
environment for the bugs to thrive.
Virtually all troubles associated with BOD testing can be traced to a disturbance in one of these key aspects, and it often helps to reflect on the importance of the pyramid when troubleshooting your analysis.
Food source
If there is no food source, there can be no BOD, since there would be no oxygen required for breakdown of the food. The logical question to ask is: What if your effluent is ultra-clean; it has no food source for the bugs? The answer is that in very clean samples (200 mLs or more of sample used) we need to add extra nutrients to ensure that bugs continue to survive.
Population of microorganisms (bugs)
On the other side of the coin, we can have a sample with a huge amount of BOD loading, but if there are no living bugs during the BOD test, then no oxygen will be utilized, and the BOD results will be unrealistically low. The consequences of this problem would be discharging a waste that may subsequently reduce available oxygen in the receiving stream, having an adverse effect on wildlife downstream.
Available oxygen
You can have bugs and a food source, but there must be oxygen for the bugs to utilize in the biodegradation process. This is one of the reasons why we stress the importance of beginning with a sample that is saturated with oxygen. With a requirement of a minimum depletion of 2 mg/L and a final DO of at least 1 mg/L, we don't have a great deal of working room with which to obtain at least one dilution which meets acceptance criteria.
BOD test is not easy
The reality is that BOD is a tough test. This is not chemistry; this is really a sort of bioassay which is more similar to whole effluent toxicity (WET) testing than any other testing. That being said, the BOD test can be reigned in and kept under control. In order to do that, one has to understand the basic concepts outlined here and on associated links. The basics are: make sure you have happy, healthy bugs and a good calibration to ensure we know precisely how much oxygen was used (accurate initial and final DO readings).
Significance of the BOD test
- Most commonly required test on WPDES discharge permits.
- Widely used in facility design planning.
- Assess waste loading on surface waters - what are impacts downstream.
- Characterized as the "the test everyone loves to hate."
Common BOD Problems
- Sampling and/or preparation issues.
- Calibration issues.
- Consistently meeting GGA limits.
- Ensuring sufficient seed activity.
- Adding the right amount of seed.
- DO membrane and probe performance.
- Sample size.
- Nitrification.
- Sample toxicity.
- Improper interpretation of results.
BOD test limitations
GGA provides flawed metrics
GGA measures accuracy at 200 ppm (which may give an indication of accuracy on influent samples) but most effluents range from 5-25 ppm. How do we know what the accuracy is at these levels which are 1-2 orders of magnitude lower than the GGA level? Additionally, the acceptance criteria for GGA are based on the mean plus/minus a single standard deviation.
- Test period is too long.
- Test is not well suited for process control.
- There is no good means to evaluate accuracy - GGA is flawed.
- The test is simply not very easy
Why BOD is still required
- None of the alternatives provide a better assessment of the bioavailability of a waste like the BOD test.
- BOD is a very complex test; however, consistent and reliable BOD results can be produced by any lab if analysts:
- use good laboratory QC practices;
- pay attention to details; and
- carefully follow the method.
SAMPLING AND SAMPLE HANDLING FOR BOD
Water and wastewater samples are biologically active, and the BOD will quickly deteriorate. Minimize the time between sampling and analysis to obtain the best BOD results. Take the following precautions when sampling and handling BOD samples.
Record the composite sampler temperature in permanent ink daily!
SAMPLING CONSIDERATIONS
- Preferably sample before any disinfection.
- If sampling after any disinfection, samples must be seeded.
HANDLING CONSIDERATIONS
- Composite samples must be kept at 0 - 6°C.
- Cool samples on ice between the composite sampler and laboratory.
- Maximum holding time is 48 hours for both grab and composite samples.
- Composite samples - this means 48 hours after the sample is removed from the auto-sampler.