BOD seed
Seed preparation and addition
De-ionized water kills - Adding a seed capsule to deionized water causes an osmotic imbalance that will kill seed organisms. The purified water —containing no salts— rushes across the bacterial cell walls. The bacteria subsequently swell up and burst (or lyse) which, if it does not kill them, will severely impair their ability to utilize oxygen.
Seed source
- Domestic WW supernatant; settled at 20° C > 1 hr but < 36 hr.
- Commercial seed (e.g., BOD seed, Polyseed)
- …may need to mix longer/differently than manufacturer recommendations
- DO NOT mix seed in distilled or deionized water!
- Effluent from a biological treatment system is NOT recommended, as it creates the potential for nitrification to occur.
Preparing seed
We recommend decanting and stirring vs. drawing individual aliquots off top. When you draw individual aliquots using a pipet, the vacuum action actually draws up some of the settled organisms, resulting in much more variable seed additions. By letting the seed settle and then carefully pouring off— or decanting— the supernatant, you ensure a much more consistent (between additions) seed.
Seed addition
- You can either:
- seed dilution water, or
- seed samples directly.
- Either practice is acceptable.
- Seeding dilution water ensures all samples seeded.
- Seeding individual samples makes calculating seed correction a much easier task.
Adding seed directly to bottles
| Estimated seed BOD | Suggested seed control volumes | Suggested seed volume per BOD bottle |
|---|---|---|
| 30 mg/L | 15, 25, 50 mLs | 6 - 10 mLs |
| 50 mg/L | 15, 25, 50 mLs | 4 - 6 mLs |
| 100 mg/L | 5, 10, 15 mLs | 2 - 3 mLs |
| 150 mg/L | 5, 10, 15 mLs | 1 - 2 mLs |
- Adjust amount of seed to BOD bottle to obtain GGA results in the 198 ± 30.5 range.
- Never pipet seed material into a dry BOD bottle.
- Always have some dilution water in first.
- Adding seed to DI water can rupture (lyse) cells.
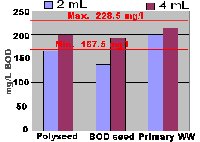
2 mLs seed doesn't always work!
Many labs use 2 mLs of seed-to-seed samples or GGA. This may be because "it's what we've always done" or because that's what the manufacturer recommends. The most important thing to remember when deciding how much seed to add is to consider GGA results. The proper amount of seed to add is that volume of seed that yields acceptable GGA performance. The graph below clearly shows that seed strength varies with seed source, and 2 mLs of seed may not work for all seed sources… and may even change between lots obtained from a single vendor.
When obtaining a new lot of seed or seed from a new source, test it out on GGA using several different volumes to best determine the amount of seed that works for THAT lot/source.
Seed correction
Individually seeded bottles
| Bottle # | DOI | DOF | Depletion | mLs of seed | DO depletion per mL seed |
|---|---|---|---|---|---|
| Seed Control 1 | 8.5 mg/L | 0.3 mg/L | 8.2 mg/L | 30 mLs | ----- |
| Seed Control 2 | 8.4 mg/L | 1.6 mg/L | 6.8 mg/L | 20 mLs | 0.34 mg/L per mL |
| Seed Control 3 | 8.4 mg/L | 4.3 mg/L | 4.1 mg/L | 10 mLs | 0.41 mg/L per mL |
Seed Control 1 is not used due to the insufficient final DO
Average the seed controls that meet depletion criteria
= (0.34 + 0.41) ÷ 2
= 0.375 mg/L DO depletion per mL seed
Thus, if 2 ml undiluted seed is added to each sample bottle,
seed correction = 0.375 mg/L DO X 2 ml seed = 0.75 mg/L
Therefore, 0.75 mg/L is subtracted from the depletion of each sample BOD depletion before calculating final BOD result.
Seeding dilution water
When seeding dilution water, there is no need for seed controls per se…a 300 mL volume of dilution water is analyzed and THAT serves as the seed control.
In the following example, the dilution water "seed control", labeled "Dilution Water Blank" here, shows an oxygen depletion of 2.4 mg/L after 5 days.
For the sample, in dilution "1", we need to correct for the depletion due to dilution water itself. Since 300 mLs depleted 2.4 mg/L, then 150 mLs would be expected to deplete 150/300 or 0.5 x 2.4 mg/L, or 1.2 mg/L.
| Sample Dilution | DOI | DOF | Depletion | mLs of sample | mLs of Dilution Water |
|---|---|---|---|---|---|
| Dilution Water Blank | 8.6 mg/L | 6.2 mg/L | 2.4 mg/L | 0 mLs | 300 mLs |
| Sample Dilution 1 | 8.5 mg/L | 1.1 mg/L | 7.4 mg/L | 150 mLs | 150 mLs |
| Sample Dilution 2 | 8.4 mg/L | 2.6 mg/L | 5.8 mg/L | 100 mLs | 200 mLs |
| Sample Dilution 3 | 8.4 mg/L | 3.9 mg/L | 4.5 mg/L | 50 mLs | 250 mLs |
Depletion due to seed:
Sample Dilution 1 = 2.4 x (150/300) = 2.4 x 0.5 = 1.2
Sample Dilution 2 = 2.4 x (200/300) = 2.4 x 0.6667 = 1.6
Sample Dilution 3 = 2.4 x (250/300) = 2.4 x 0.8333 = 2.0
BOD [Sample Dilution 1 = (7.4 - 1.2) × (300 ÷ 150)
= 6.2 × 2 = 12.4
BOD [Sample Dilution 2] = (5.8 - 1.6) × (300 ÷ 100)
= 4.2 × 3 = 12.6
BOD [Sample Dilution 3] = (4.5 - 2.0) × (300 ÷ 50)
= 2.3 × 6.0 = 13.8
Average BOD of three dilutions =
= 12.4 + 12.6 + 13.8 ÷ 3
= 38.8 ÷ 3
= 12.9
The hardest part of choosing to seed dilution water is choosing how much seed to add to dilution water to allow enough depletion in diluted samples. For example…if 300 mLs of dilution water only depleted 1.0 mg/L, that would not be sufficient, because the same rules as for samples apply. In other words, you need to prepare at least two dilutions, and the goals is to come up with at least two dilutions that meet method depletion requirements. You need to add enough seed organisms to the dilution water such that 300 mLs produces a drop of at least 2.0 mg/L (and also have a DO residual of not less than 1.0 mg/L).
The other thing to remember is that if dilution water is seeded, ALL dilutions must be individually adjusted for seed correction.
Seed controls
The most critical thing to remember with seed controls is that the purpose of analyzing seed controls is to determine the BOD of your seed material. Therefore, seed controls MUST be subject to the same criteria as samples, including number of dilutions, and depletion criteria.
- Need at least one dilutions
- Best to do 3 dilutions
- Calculate seed correction factor
- Many continue to view seed depletion criteria of 0.6 to 1.0 mg/L as a requirement. This is simply not the case. Standard Methods has updated the language to clarify this issue. The intent is to adjust seed volume to the point at which GGA acceptance criteria are met. If GGA results are low, you should add more seed. If GGA results are too high, reduce the seed volume and/or evaluate potential sources of contamination.
Seeding summary
- It has been reported that commercial seeds yield low GGA results. Good GGA results were achieved when larger volumes of the commercial seed were used.
- Remember: the 0.6 to 1.0 mg/L seed depletion is guidance!
- Domestic primary wastewater seed seemed to provide the most consistent GGA results.
- Note however that any wastewater seed strength can change with conditions (e.g., rainfall, I&I).