Method Details
The following are the critical steps in preparing and analyzing samples for BOD testing. The BOD test is a method-defined (empirical) technique. Consequently, the method must be followed carefully, and must be performed consistently from day to day and analyst to analyst. Potential errors can be encountered in each of the following steps in the BOD test.
- Prepare dilution water
- Prepare seed
- Perform the preliminary testing
- Determine dilutions required
- Measure out samples
- Fill bottles three-quarters full of dilution water
- Add seed to those that need it
- Top-off samples with dilution water
- Measure initial DO (DOi)
- Incubate 5 days (± 4-6 hrs)
- Measure Final DO (DOF)
- Determine BOD
- Evaluate the results of samples, GGA, blanks and duplicates and report.
Determining appropriate dilutions
Because the BOD test is a bioassay technique, it is inherently less accurate and precise than analyte-specific tests such as ammonia and total phosphorus. The BOD test is also limited by capacity of water to hold oxygen. Consequently, the analyst must prepare more than one dilution to ensure the BOD depletion criteria are met (e.g., at least 2 mg/L DO consumed and 1 mg/L residual DO during the 5-day test period).
- Recommend at least two dilutions (preferably > 3).
- The more dilutions used, the easier it is to identify toxicity problems!
- *** The 20th Ed. of Standard Methods requires at least 2 dilutions meet the depletion criteria.***
- WWTPs tend to be more familiar with waste (it is theirs, after all!), thus can get away with fewer dilutions.
- Commercial labs tend to use more dilutions since they receive many samples and may not be as familiar with the samples they receive.
- Use dilutions which will result in adequate depletion.
- Need to use dilutions which will not over-deplete.
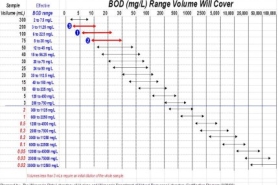
BOD volume estimation chart
of Hygiene and Wisconsin Department of
Natural Resources Laboratory Certification
Program (9/28/99).
Click for larger image.
Assumptions: 8.5 mg/L DOI; meets method depletion requirements
Example: Sample BOD is expected to be about 5 to 25 mg/L
One of the first steps in the BOD testing is to determine the volume needed to prepare dilutions for the 5-day incubation period. The above chart is a convenient way to graphically determine the dilutions (sample volumes) needed for the BOD test. The chart was prepared based on the assumption that the initial DO of the dilution water will be 8.5 mg/L and, there must be at least 2 mg/L depletion during the 5-day test period and at least 1 mg/L residue DO remaining.
The idea is to settle on the best dilution which fits the expected BOD range of the sample (1) and then prepare one lower dilution [more sample] (3) and one greater dilution [less sample] (2).
For example, if the expected BOD is between 5 and 25 mg/L, use sample volumes of 5, 10 and 20 mL. The red horizontal bars show the BOD each sample volume will cover. This type of chart can easily be posted at the BOD workstation as an aid to analysts and technicians.
More on determining appropriate dilutions
| Estimated BOD5 | Suggested Sample Volumes |
|---|---|
| < 5 mg/L | 200, 250, 300 mLs |
| < 10 mg/L | 100, 150, 200 mLs |
| 10 - 30 mg/L | 25, 50, 100 mLs |
| 30 - 60 mg/L | 15, 25, 50 mLs |
| 60 - 90 | 10, 15, 25 mLs |
| 90 - 150 mg/L | 5, 10, 15 mLs |
| 150 - 300 mg/L | 3, 5, 10 mLs |
| 300 - 750 mg/L | 1, 3, 5 mLs *** |
| 750 - 1500 mg/L | 0.5, 1, 3 mLs *** |
| 1500 - 2500 mg/L | 0.25, 0.5, 1 mLs *** |
The table above is another aid that may be used by analysts and technicians to determine sample dilution. Either the graphic or tabular dilution guide is recommended to provide consistency among analysts.
Making initial dilutions (if you need to use < 3 mLs)
If less than 3 mL of samples volume is used (<1 percent dilution), a preliminary dilution is required prior to pipetting the sampling into the BOD bottle. This is best done by preparing a simple dilution and then pipetting a portion of the diluted sample into the BOD bottle. For example, if sample volumes of 0.5, 1.0 and 2.0 mL of sample are needed, make a 10-fold preliminary dilution. Prepare the 10-fold dilution by pipetting 10 mL of well mixed sample into a 100 mL volumetric flask (or 10 mL graduated cylinder) containing about 50 mL of BOD dilution water. Bring the flask to 100 mL by adding additional BOD dilution water and then mix thoroughly. The below table illustrates the resulting volumes. Remember: the greater the original sample volume used to make an initial dilution, the more representative the dilution will be of the original sample!
Making an initial 10-fold dilution
- 10 mLs sample to 100 mLs total volume (with dilution water)
- 25 mLs sample to 250 mLs total volume (with dilution water)
- 50 mLs sample to 500 mLs total volume (with dilution water)
- 100 mLs sample to 1000 mLs total volume (with dilution water)
Make all dilutions with large-bore volumetric pipets and flasks!
| mLs of 10X dilution | mLs of original sample |
|---|---|
| 5 | 0.5 |
| 10 | 1.0 |
| 20 | 2.0 |
| 25 | 2. |
| 50 | 5.0 |
General BOD tips
The following are items of important consideration when preparing and performing the BOD test.
- ROTATE BOD bottles!
- NEVER use the same bottles for blanks and GGA. Rotate bottles so you can prove your cleaning technique is satisfactory.
- If you always use the sample bottles for your blank, you will not be able to prove the other bottles are clean.
- Samples must be brought to 20 ± 3 °C before BOD testing.
- Auditors will expect that the lab temperature should also be maintained between 17-23°C if you expect to prepare samples for BOD testing (Standard Methods, 20th Ed.)
- Record the lab temperature and barometric pressure daily.
- You must record the barometric pressure and air temperature of your laboratory if the water-saturated air or air-saturated water techniques are used to calibrate your DO probe.
- You also need both measurements in order to determine whether or not samples are super-saturated and require pre-treatment.
Measuring out samples
One of the most critical operations, and an often-overlooked process, in the BOD test is measuring out and transferring the sample to the BOD bottle. Failure to use good technique can cause erratic results. BOD results can also exhibit what many call the toxic effect or sliding BOD results.
Use only wide-bore, volumetric pipets for BOD testing. These pipets are designed "to contain" (TC) a specific sample volume and are intended to have the sample blown out after the sample is delivered to the BOD bottle. Do not use narrow-bore volumetric or serological pipets for samples. The narrow bore will act as a filter and will prevent particulates from entering the pipet. This will result in poor sub-sampling and low BOD results.
Wide-bore volumetric pipets are available in a variety of volumes from many scientific specialty companies. However, we recognize that there are few volume choices below 10 mL. Consequently, it may be necessary to use wide-bore serological pipets to cover these volumes. Remember, if you must use serological pipets, only use wide-bore types, use them once and refill between pipettings. For example, if you need 3 mL and 6 mL volumes, don't fill a 10 mL serological pipet and sequentially deliver the 3 mL and 6 mL without refilling the pipet. Remember, particles will settle in the pipet during the sample delivery step. There likely be more particles in the second volume delivered so you will likely have poor agreement between dilutions (think: failure of replicates!). If you need 3 mL and 6 mL, refill the pipet between each of these pipettings.
The BOD test is a tough test to do properly. Don't make life difficult for yourself by using poor pipetting or sub-sampling technique.
- Do not use more than one pipet for a given sample
- Example: If using 25 mLs, don't use both a 5 mL and a 20 mL pipet... Use ONE 25 mL pipet
- DON'T fill a pipet twice to obtain a certain volume
- Example: If using 200 mLs, don't pipet twice with a 100 mL pipet.
Use a graduated cylinder. - Remember, error is additive; each time you measure use a pipet or graduated cylinder, there is error. If you pipet twice, you error is double!
- Keep it simple— only fill your pipet or graduated cylinder once!
- Serological pipets
- Ensure that they are wide-bore, and
- Use them once. Re-fill after each pipetting.
- Graduated cylinders (> 100 mL)
- DON'T agonize over "getting exactly to the mark". Use the "rapid attack" approach:
- Pour quickly
- Get as close to target volume as you can;
- Record actual volume used
- The longer you agonize over the volume, the more opportunity the particulates in the sample have to settle out. This can bias your sample which can result in either a higher or lower BOD results.
Always re-mix the sample between pipetting out each dilution. If you mix only initially, particles will settle out the longer the sample bottle sits. If you pipet slowly, solids are settling all the while. Each subsequent pipetting will contain fewer solids if pipetting from the top, or more solids if pipetting from the bottom of the bottle.
Large sample volumes require extra nutrients
When larger sample volumes are used to prepare BOD dilutions (>200 mL), it will be necessary to add extra nutrients. Use the following guidance to determine the amount of supplemental nutrients to add:
CURRENT GUIDANCE: Standard Methods
When a bottle contains more than 67% of the sample (> 200 mL) after dilution, nutrients may be limited and subsequently reduce biological activity. The resulting BOD will be biased low. In such samples, add the nutrient/buffer solutions (3a through 3e) directly to each BOD bottle at a rate of 1 mL/L (0.33 mL/300-mL bottle) or use commercially prepared solutions/pillows designed to dose the appropriate bottle size.
When individual nutrient pillows are used, it's OK to use dilution water that may contain some nutrients already.
Make life easier— use the nutrient pillows designed for individual 300 mL BOD bottle.
Measuring initial DO
Below are important considerations when making the initial DO measurements for the BOD test.
- It's a good idea to warm up the meter and calibrate first.
- If the meter has already been used earlier, re-check the calibration.
- Don't let samples sit too long between adding dilution water and the initial DO measurements.
- Standard Methods requires measuring the initial DO no longer than 30 minutes after adding the dilution water.
- Impact of a long delay on samples with rapid or instantaneous demand—
- - you will lose that instantaneous measure.
- - if assess user fees, instantaneous BOD can result in lower fees.
- Must actually measure the DO of each dilution (vs. measuring initial sample DO and reporting for each dilution).
Incubating samples
The incubation step is a critical part of the BOD test. The 5-day incubation period was originally established by early BOD pioneers based on the length of time it took water to flow from London, England, down the Thames River to the North Sea. Although this may seem like an arbitrary time, it has been internationally adopted as the "standard" incubation period for the BOD test. The following are critical considerations related to the incubation period.
- Incubate for 5-days (hence the term BOD5 ) ± 4-6 hours. Anything beyond that and your results may be questioned.
- At 20 ± 1 °C (In the dark). Document temperature each day samples are in progress.
- Fill water seals with dilution water; cap to reduce evaporation.
- Check daily, add water to seals if necessary.
- Before removing stoppers, pour off the water in the seals.
| Day in) | Read out on |
|---|---|
| Monday | Saturday |
| Tuesday | Sunday |
| Wednesday | Monday |
| Thursday | Tuesday |
| Friday | Wednesday |
| Saturday | Thursday |
| Sunday | Friday |
Due to the 5-day testing period, certain samples require that set-ups and read-outs of results be performed by different individuals.
Calculating BOD
The final step in the test is to perform the needed calculations and to evaluate the final BOD results.
Care must be taken to consider all of the below items:
- Dilutions meet depletion criteria?
- Residual DO at least 1 mg/L
- DO depletion at least 2 mg/L
- Average dilutions meeting depletion criteria.
- Check for sample toxicity
Copyright 2006. University of Wisconsin Board of Regents.
Unauthorized use prohibited without the expressed written consent of the UW, State Laboratory of Hygiene and the DNR Laboratory Certification & Registration Program.
- Contact information
- For information on the Lab Certification program, contact:
- Tom Trainor
Program chemist
Certification Services
DNR LabCert mailbox