BOD dilution water
BOD dilution water nutrient solutions can be a source of contamination. If you prepare your own solution, make sure you store the phosphate buffer in a refrigerator. Discard any solution if it becomes cloudy or you observe any "chunks" floating in the solution. Using single-use nutrient buffer pillows will avoid many of these pitfalls.
Nutrient Solutions:
- Magnesium sulfate solution: 22.5 g MgSO4•7H20. Dilute to 1 L.
- Calcium chloride solution: 27.5 g CaCl2. Dilute to 1 L.
- Ferric Chloride solution: 0.25 g FeCl3•6H20. Dilute to 1 L.
- Phosphate buffer: 8.5 g KH2P04, 21.75 g K2HP04, 33.4 g Na2HP04•7H20, and 1.7 g NH4Cl. Dilute to 1 L.
The pH should be 7.2.
Store in 4°C refrigerator.
Check before each use for contamination (discard any reagent with growth).
- Add 1 mL each of PO4 buffer; MgSO4, CaCl2, and FeCl3 per Liter or the contents of one buffer pillow (buy the right size!).
- Before use bring dilution water temperature to 20 ± 1°C.
- Saturate with DO:
- shake or aerate with organic-free filtered air
- store in cotton-plugged bottles "long enough to become saturated"
Dilution water misconceptions
There are many misconceptions regarding dilution water preparation. Many have taught that dilution water cannot be mixed, aerated or shaken immediately before use because the water will be supersaturated. This is not true. Dilution water at room temperature (17-23°C) can be rigorously mixed, shaken or even aerated with compressed air minutes before use without fear of super-saturation. Excess oxygen will physically dissipate almost immediately. In fact, to the contrary... shaking dilution water after it has equilibrated to room temperature is required to bring the water to saturation.
Here are some simple tips for dilution water preparation:
Dilution water should be prepared immediately before use. Without the phosphate buffer, you can prepare dilution water days/weeks ahead of time. Phosphate buffer is a key reagent because phosphorus is the limiting nutrient in stimulating growth, so it must be added the day the water is used.
- Use only high-quality distilled or deionized water.
- Allow water to equilibrate for longer than 24 hours at 20°C in a temperature-controlled room (17-23°C) before use.
- Add the nutrient buffer solutions the day the water is to be used.
- Avoid contaminating the water while allowing the water to become oxygenated. Always cover the bottle with a cotton plug, sponge or clean paper towel.
- If you use compressed air, filter the air.
- Do not allow anything but glass or Teflon to come in contact with the water.
Never allow "fish tank" (tygon) tubing or air stones to come in contact with the water. Tygon will leach oxygen-demanding material over time and air stones provide an excellent surface for bacterial growth. If they are used, you may inoculate your BOD dilution water with bacteria.
If you wish to age your dilution water, do not add the single-use nutrient buffers until the day the water is used. Please note that you should not need to age lab reagent water if it is prepared properly.
Excessive depletion in dilution water
Excessive DO depletion in BOD blanks is one of the most common problems that plague all wastewater testing laboratories. Even the best labs occasionally have problems meeting the DO depletion in the BOD blanks (< 0.2 mg/L). The following are the most common causes for excessive depletion in blanks:
- Tubing is constructed of oxygen-demand leaching material
- Correct tubing not being used throughout the lab
- Slime growth in delivery tubing
- Poor water quality/improperly maintained system
- Poorly cleaned BOD bottles or dilution water storage container. NOTE: Glass is best!
- Contamination during aeration
- Poorly calibrated DO Probe
Solving dilution water quality problems
Bottled water
Generally, avoid using "grocery store" distilled water. This water is stored in plastic bottles, which can leach oxygen-demanding materials. Some analysts have had great luck using "grocery store" distilled water. If you have good luck with a particular brand, don't change. However, be aware that you can't control how the water is stored. If the water sits on a shelf for several months in a hot warehouse, it's going to be a problem. Nothing leaches organic matter from plastic like hot water.
Those that have successfully used "grocery store" water generally have a system. Some will go directly to the factory and buy fresh water. They then date the water, store it in a cool, dry place and discard the water after it is a certain age (e.g., expiration date). Bottom Line: Use it if it's working for you; but if it isn't, consider other alternatives.
Aging dilution water
You should not have to age dilution water if the water is prepared properly. If you have to age to improve quality, you should be concentrating your efforts on improving the preparation process.
Deodorizers and Dilution Water
Avoid using auto-dispensing deodorizers in the laboratory. You may be tempted to use deodorizers because wastewater labs can have a bit of an odor. However, don't use them! They typically use alcohol as a carrier which has a very high BOD.
Water purification systems
All lab reagent water systems, much like expensive instruments, are prone to problems if they are not maintained properly. Follow the manufacturer's recommendations for cleaning and disinfecting stills, water polishers and deionizer systems.
Simple deionizer systems can work well but can quickly be overgrown with bacteria and mold and can leach organics. Again, regular scheduled maintenance is the key to keeping a system working optimally.
A simple deionizer system can and has worked very well for many laboratories. However, you must use quality virgin or nuclear-grade resins. Poorer grade or frequently 're-generated' household softener resins WILL leach oxygen-demanding material and will NOT work for BOD testing. Don't let a manufacturer talk you into lower quality resins to save money. This is a "penny wise and dollar foolish" approach. Don't sacrifice quality for a few bucks.
Activated charcoal in deionizer systems are also prone to contamination problems. They can quickly become contaminated with bacteria and mold and can slough-off oxygen demanding material.
Chlorinated water directly feeding ion exchange systems can breakdown and leach oxygen demanding material. The solution is to pass the water through activated charcoal cartridge prior to resin.
SLH's dilution water experiences
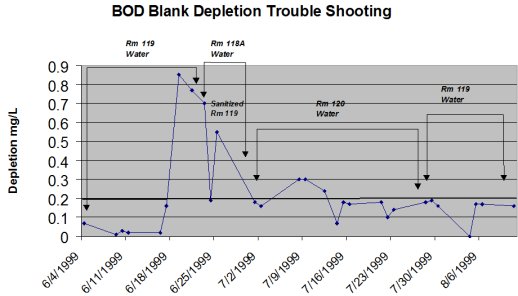
The Wisconsin State Laboratory of Hygiene (WSLH) built a state-of-the-art laboratory in 1999. They also installed a state-of-the-art laboratory reagent water system. However, the manufacturer sold them poorer grade anion and cation exchange resins, which allowed them to be "low bidder." The WSLH was unable to get BOD blanks to pass the depletion criteria (i.e., <0.2 mg/L) using this water. They spent months troubleshooting the water system. After many months of systematically troubleshooting the system, the WSLH discovered the problem and switched to virgin or nuclear grade resins. The graph illustrates the problems they experienced.
So be aware that even the best systems will not produce water good enough for BOD testing unless the ion exchange resins are used. End-of-line water polishing units will NOT remove all of the organics leached from the poorer quality resin even though manufactures may claim they will. Quality lab reagent water is the foundation of all laboratories. Remember, you get what you pay for. Don't scrimp and shortchange your lab by buying a "cheap" system.
Dilution water- simplest solutions
There are a number of simple solutions to obtaining quality lab reagent water to prepare BOD dilution water.
- Obtain water from another laboratory or vendor.
- Probably the easiest approach is to obtain water from another laboratory that has a track record of producing consistently high-quality BOD dilution water. It may be easier to haul water once a week than maintaining a lab water system, particularly if you have a small laboratory.
- Purchase water from a source that has proven success.
- You may also find a commercial source of water that is of consistent quality. If you find a particular brand and supplier that works for you, stick with it! Don't fix it if it isn't broken.
- Buy an all-glass laboratory still and distill your own water.
- If you only need a limited amount of water (e.g., < 5 gallons per week), consider buying an all glass still. They are less costly than other water purification systems and tend to produce quality reagent water provided they are maintained properly.
- Buy a bench-top water reverse osmosis (RO) and polisher combo that will produce ASTM Type I water.
- These systems work well. However, they tend to be expensive (more than $1,000) and require regular maintenance to be effective.
Solving: glassware cleanliness concerns
- Use a good lab-grade non-phosphate detergent and bleach.
- Rinse thoroughly with tap water followed by distilled water.
- Allow to dry before storing.
- Always cover glassware and store in a clean, dry place.
- Commercial lab dishwashers with non-phosphate, lab grade detergents and acid rinse solutions (non-phosphate) will also work well.
- Use BOD QC samples (blanks, duplicates and GGA) to evaluate washing effectiveness.
Alternate glassware cleaning protocol without bleach
Bleach-free alternate cleaning method
(HCl) and bleach: It will produce
poisonous chlorine gas.
- Use a good laboratory grade non-phosphate detergent.
- Rinse thoroughly with tap water followed by dilute HCl (10% solution; 100 mL HCl per liter of water).
- Rinse again with tap water followed by distilled water.
- Allow to dry before storing.
- Always cover glassware and store in a clean, dry place.